“耀杰不凡”致敬幕后抗疫英雄推文解读活动
第二刊:“肛拭子”频频上热门?伯杰医疗邀你一起知其所以然
近日,采集“肛拭子样本”用于新冠筛查的新闻成为大家热议的焦点,事件的起因是1月20日,北京新冠防控新闻发布会介绍,其已对大兴某新发病例所在学校全体学生、教职工等全部进行鼻咽拭子、口咽拭子、肛拭子及血清检测。
那么问题来了,此次北京大兴为什么在筛查中增加了“肛拭子”检测?其实,有关样本类型检出率这一议题,早先便有众多学者对其进行过讨论。2020年8月公布的《新型冠状病毒肺炎诊疗方案(试行第八版)》中也明确表示,可以在粪便、尿液中可分离到新冠病毒。


鼻咽拭子、口咽拭子、痰液、支气管灌洗液及肛拭子这些不同类型的标本与检测结果之间到底存在着什么样的联系呢?不同的病程进展情况下,上述各样本类型是否存在检出差异?且听大咖见解,一一道来~
本研究成果由深圳市第三人民医院的刘映霞教授、刘磊教授和中科院深圳先进技术研究院的李亮教授团队在2020年5月16日发表于胃肠病学和肝病专业领域国际排名第一的医学杂志《胃肠病学(Gastroenterology)》,研究主题为新冠肺炎患者的肠道排毒特征及规律。


图1. 患者粪便中 SARS-CoV-2在病程不同时间(周)的检测情况。
解读文献2:Laboratory Diagnosis and Monitoring the Viral Shedding of SARS-CoV-2 Infection

本期研究收集了410例患者的3,552份呼吸道标本,包括559份口咽拭子,2,231份鼻咽拭子,696份痰标本和66 份支气管肺泡灌洗液(BALF)样本。在这些患者中,90例为重症患者,320例为轻度和中度患者。
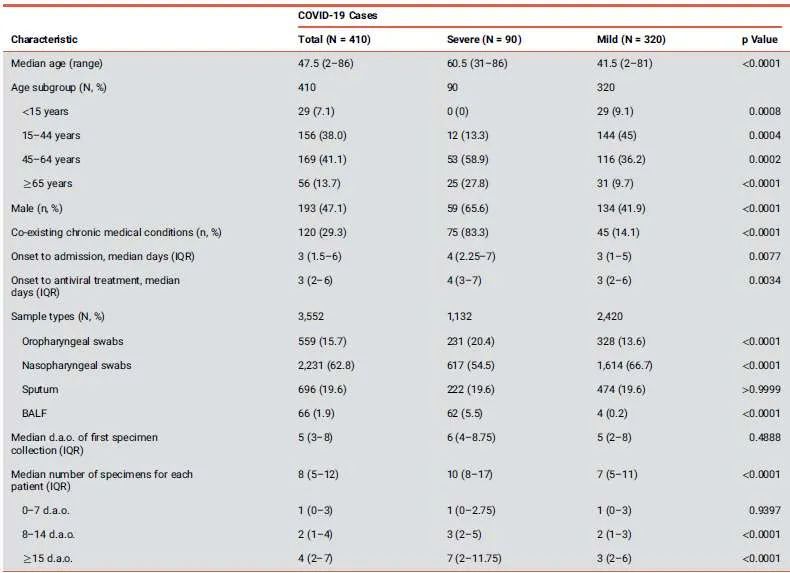
图2. 患者状况分布图
不同呼吸道样本中检测新冠病原体
上,下呼吸道的不同检测结果
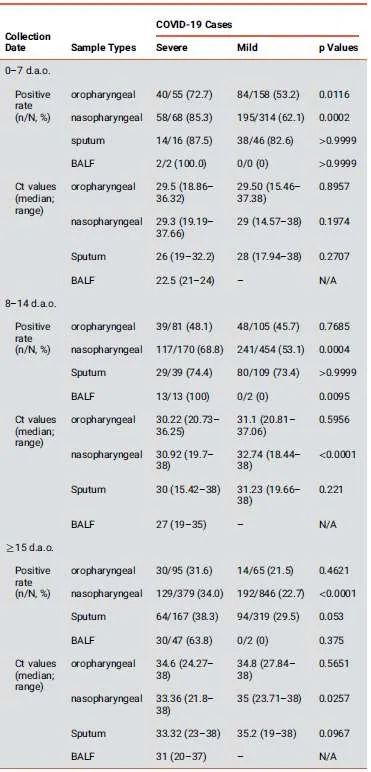
图3. RNA病原体的检测状况

1.多种样本检测结果互为补充,能够有效防止漏检:根据实验结果,新冠不同疾病程度患者的痰样本、鼻咽拭子、口咽拭子阳性率不同,痰液的检出率相对较高。
2.重症病例上呼吸道样本检出率低,需联合其他辅助手段:经过上,下呼吸道的样本检测,一些重症病例的上呼吸道样本中未检测出病毒RNA但在痰液样本中显示为阳性。由此可见,尽管未在上呼吸道样本中检测到病毒RNA,但不应将那些具有接触史和临床症状的可疑病例排除。在这种情况下,CT扫描可能为诊断COVID-19提供重要证据。
3.下呼吸道病毒脱落时间更长:研究发现,下呼吸道的病毒脱落时间可持续长达46天,这比先前发现的上呼吸道样本的病毒脱落时间更长。在制定治疗和抗击病毒传播的策略时,应谨慎对待新冠病毒的异常病毒脱落情况。

图4. RNA病毒的脱落持续时长
本文中所提及的学术研究所使用的检测试剂,来自于上海伯杰医疗科技有限公司的新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法)。此番能够在科研合作中出一份力,伯杰医疗深感荣幸。成立以来,伯杰医疗一直专注于传染性病原体的诊断,秉承“勇于创新,质量为先”的方针。助力检验科研事业的发展,伯杰医疗责无旁贷!
参考文献:
1. Zhu, N., Zhang, D., Wang, W., et al. (2020). A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382, 727–733.
2. Tan, W., Zhao, X., Ma, X., et al. (2020). A novel coronavirus genome identified in a cluster of pneumonia cases—Wuhan, China 2019–2020. China CDC Weekly 2, 61–62.
3. Xu, X.W., Wu, X.X., Jiang, X.G., et al. (2020). Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 368, m606, https://doi.org/10.1136/bmj.m606.
4. Wang, D., Hu, B., Hu, C., et al. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323, 1061–1069.
5. Huang, C., Wang, Y., Li, X., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506.
6. Guan, W.J., Ni, Z.Y., Hu, Y., et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720.
7. Chan, J.F., Yuan, S., Kok, K.H., et al. (2020). A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395, 514–523.
8. Xu, Z., Shi, L., Wang, Y., et al. (2020). Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 8, 420–422.
9. Rothe, C., Schunk, M., Sothmann, P., et al. (2020). Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 382, 970–971.
10. Phan, L.T., Nguyen, T.V., Luong, Q.C., et al. (2020). Importation and human-to-human transmission of a novel coronavirus in Vietnam. N. Engl. J. Med. 382, 872–874.
12. Lei, J., Li, J., Li, X., et al. (2020). CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295, 18.
13. Li, Q., Guan, X., Wu, P., et al. (2020). Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 382, 1199–1207.
14. Mackay, I.M., and Arden, K.E. (2015). MERS coronavirus: diagnostics, epidemiology and transmission. Virol. J. 12, 222, https://doi.org/10.1186/s12985-015-0439-5.
15. Chan, P.K.S., To, W.K., Ng, K.C., et al. (2004). Laboratory diagnosis of SARS. Emerg. Infect. Dis. 10, 825–831.
16. Zhang, W., Du, R.-H., Li, B., et al. (2020). Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microbes Infect. 9, 386–389.
17. Zhang, J., Wang, S., and Xue, Y. (2020). Fecal specimen diagnosis 2019 novel coronavirus- infected pneumonia. J. Med. Virol. 92, 680–682.
18. Xiao, F., Tang, M., Zheng, X., et al. (2020). Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 158, 1831–1833.e3.
19. Wang, W., Xu, Y., Gao, R., et al. (2020). Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323, 1843–1844.
20. Ling, Y., Xu, S., Lin, Y., et al. (2020). Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med. J. (Engl). 133, 1039–1043.
21. Cai, J., Xu, J., Lin, D., et al. (2020). A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin. Infect. Dis. 71, 1547–1551.
22. Xu, D., Zhang, Z., Jin, L., et al. (2005). Persistent shedding of viable SARS-CoV in urine and stool of SARS patients during the convalescent phase. Eur. J. Clin. Microbiol. Infect. Dis. 24, 165–171.
23. Zhao, F., Yang, Y., Wang, Z., et al. (2020). The time sequences of oral and fecal viral shedding of coronavirus disease 2019 (COVID-19) patients. Gastroenterology 159, 1158–1160.
24. Yao, X., He, Z., Li, T., et al. (2020). Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 30, 541–543.
25. Chan, J.F.W., Lau, S.K.P., To, K.K.W., et al. (2015). Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 28, 465–522.
26. Cheng, V.C.C., Lau, S.K.P., Woo, P.C.Y., et al. (2007). Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 20, 660–694.
27. He, X., Lau, E.H.Y., Wu, P., et al. (2020). Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 26, 672–675.
28. Zhou, F., Yu, T., Du, R., et al. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062.
29. Yang, Y., Shen, C., Li, J., et al. (2020). Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy Clin. Immunol. 146, 119–127.e4.
30. Yang, Y., Wong, G., Yang, L., et al. (2019). Comparison between human infections caused by highly and low pathogenic H7N9 avian influenza viruses in Wave Five: clinical and virological findings. J. Infect. 78, 241–248.
31. Bi, Y., Tan, S., Yang, Y., et al. (2019). Clinical and immunological characteristics of human infections with H5N6 avian influenza virus. Clin. Infect. Dis. 68, 1100–1109.
32. Liu, Y., Zhang, C., Huang, F., et al. (2020). Elevated plasma level of selective cytokines in COVID-19 patients reflect viral load and lung injury. Natl. Sci. Rev. 7, 1003–1011.
上海伯杰医疗科技有限公司是一家致力于感染性病原体分子诊断试剂研发和应用,深耕于多重荧光PCR诊断试剂和痕量病毒二代测序试剂及相关服务的国家高新技术企业。公司围绕感染性病原体这一主线,从诊断试剂、诊断仪器、测序服务和医检所服务等多个面提供全套解决方案。公司秉承“勇于创新,质量为先”的方针,为医疗机构、疾控公卫、高校科研等合作伙伴提供优质产品与服务。

全国客服电话:400-860-3688












.jpg)